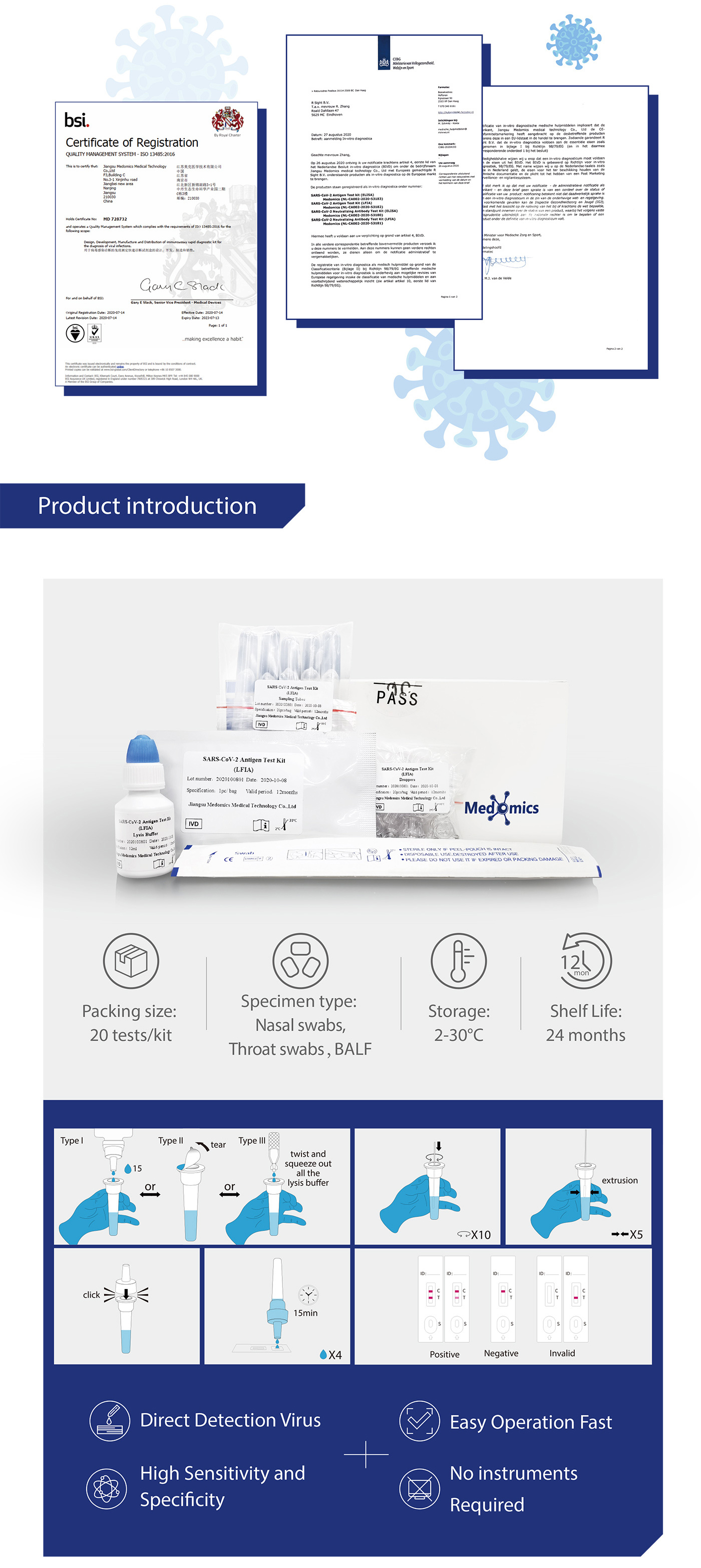
SARS-CoV-2 antigen Test Kit (LFIA) Instructions For Use
Product Name:SARS-CoV-2 antigen Test Kit (LFIA)
Product Types And Specifications
Type: IVNNI Test assette: 1pc/bag Kit: 1 pc/box
Intended Use
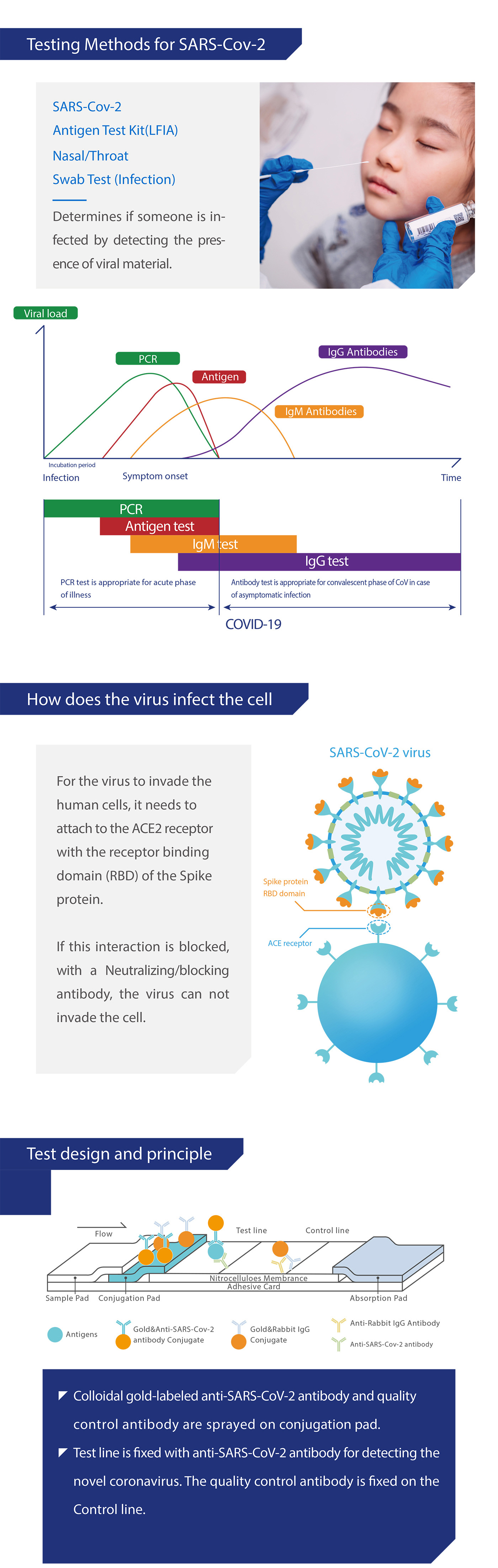
Medomics SARS-CoV-2 antigen Test Kit (LFIA) is used to qualitatively detect SARS-CoV-2 in human samples in vitro.Coronavirus (CoV) belongs to the order Nidovirales under the Coronaviridae family with 4 genera: a, β, y and δ. The a and β genera are only pathogenic to mammals, while γ and δ genera mainly cause bird infections. CoV is mainly transmitted through direct contact with secretions or through aerosols and droplets. There is also evidence supporting fecal-oral transmission.
7 kinds of human coronaviruses (HCoV) that cause human respiratory diseases have been identifled so far, including: HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1,SARS-CoV, MERS-CoV and SARS-CoV-2. SARS-CoV-2 is one of the most contagious viral pathogens that cause human respiratory tract infections (RT). Currently, the patients infected by novel coronavirus are the main source of infection.Asymptomatic infected people can also be an infectious source. Based on the current epidemiologi-cal investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The clinical manifestations include fever, fatigue, cough and other symptoms, accompanied by dyspnea,which can rapidly develop into life-threatening severe pneumonia, respiratory failure, acute respiratory vesicle syndrome, septic shock, multiple organ failure, and severe metabolic acid-base imbalance.
Test Principle
Medomics SARS-CoV-2 antigen Test Kit (LFIA) detects the SARS-CoV-2 with colloidal gold immunochromatography using a double antibody sandwich assay. The test cassette contains (1) colloidal gold-labeled anti-SARS-CoV-2 antibody, (2) one detection T line and one quality control C line fixed on a nitrocellulose membrane. T line is fixed with another anti-SARS-CoV-2 antibody for detecting the SARS-CoV-2. The quality control antibody is fxed on the C line.
When the appropriate amount of test sample treated with lysis buffer is added to the sample well of the test cassette, the sample will move forward along the test strip via capillary action. If the sample contains the SARS-CoV-2 antigens and concentration of antigens is higher than the limit of detection, the antigens will bind to the colloidal gold-labeled anti-SARS-CoV-2 antibody. The immune complex will be captured by another anti-SARS-CoV-2 antibody immobilized on the membrane, forming a red T line and indicating a positive result for the SARS-CoV-2. If the sample contains no SARS-CoV-2 antigens or the antigen concentration is lower than the limit of detection,a negative result is displayed.
Additionally, the test cassette also contains a quality control C line. Regardless of what antigens are present, the C line should appear to indicate that the sample has been transported properly through the membrane. If the C line does not appear, it indicates that the test result is invalid and the sample is required to retest.
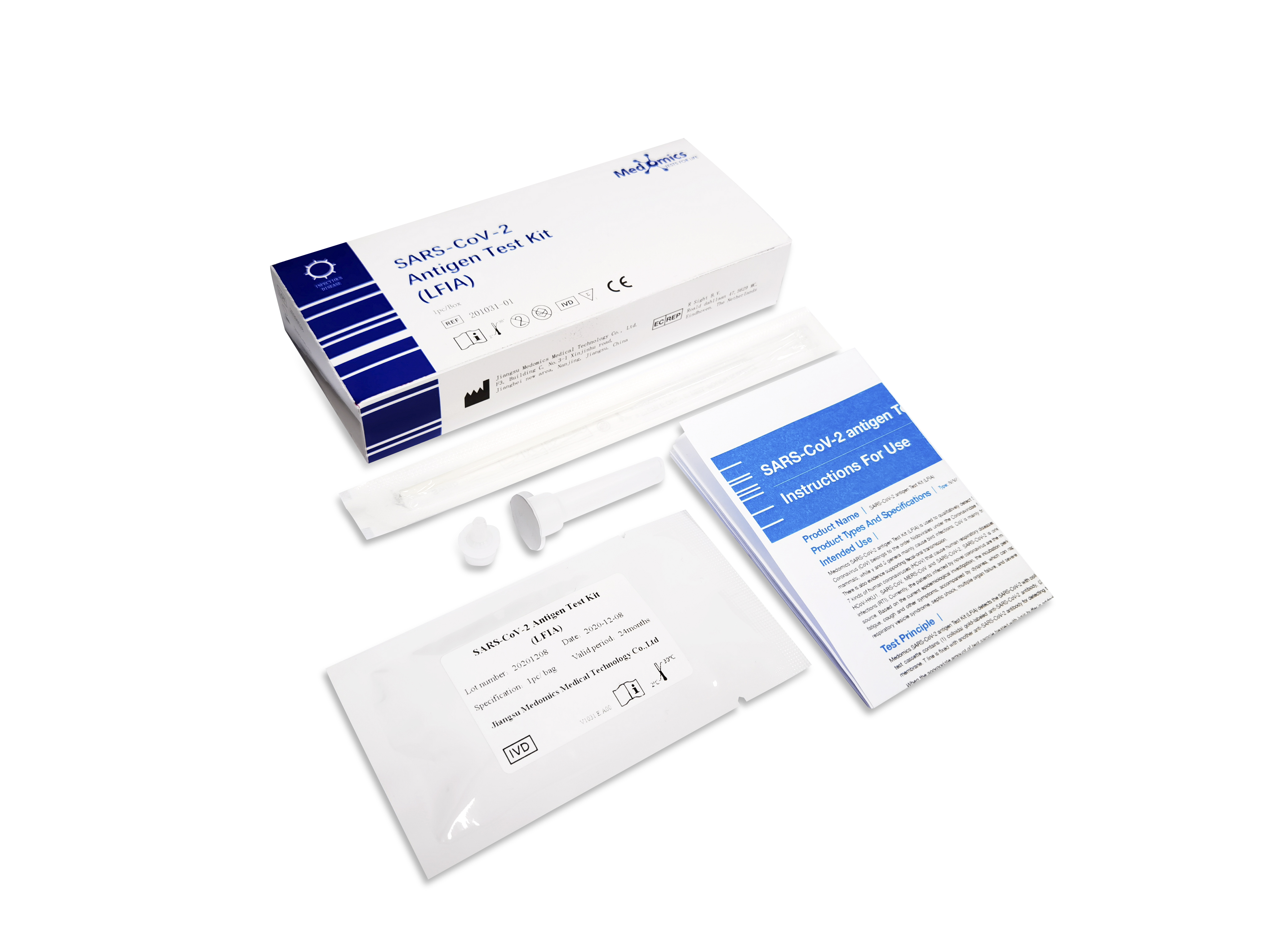
Contents of the Kit
Type IV: 1 Test Cassette |1 Oral Swab | 1 Lysis Buffer |1 Dropper | 1 Package Insert
Type V: 1 Test Cassette | 1 Saliva Collector | 1 Pipette | 1 Lysis Buffer | 1 Dropper | 1 Package Insert
Type VI: 1 Test Cassette| 1 Nasal Swab| 1 Lysis Buffer | 1 Dropper| 1 Package Insert
. Test cassette contains test strip, cassette, desiccant. The test strip contains colloidal gold-labeled anti-SARS-CoV-2 antibody, nitrocellulose membrane (C line fxed with goat-anti-mouse lgG polyclonal antibody, and T line fixed with another anti-SARS-CoV-2 antibody)
Warnings and Precautions
●This test kit is used for individuals 14 years and order.
●This test kit is used for in vitro diagnosis only.
●This test kit is for professional, non-laboratory, and at-home use.
●This test kit should be used within 1 hour after opening the package, and samples from transport media will reduce sensitivity. The test cassette should not be used if being wet or polluted.
●Proper protection should be taken during testing to avoid splashing when adding sample.
●Dispose of all used or damaged test cassettes, sampling tubes, droppers, swabs,or other kit components as biohazardous materials.
●Negative results do not rule out SARS-CoV-2 infection,particularly in those who have been in contact with the virus.
Storage Instructions
The test kit should be stored away from direct sunlight at 2°C to 30°C with a shelf-life of 24 months. Do not freeze.
Sample Requirements
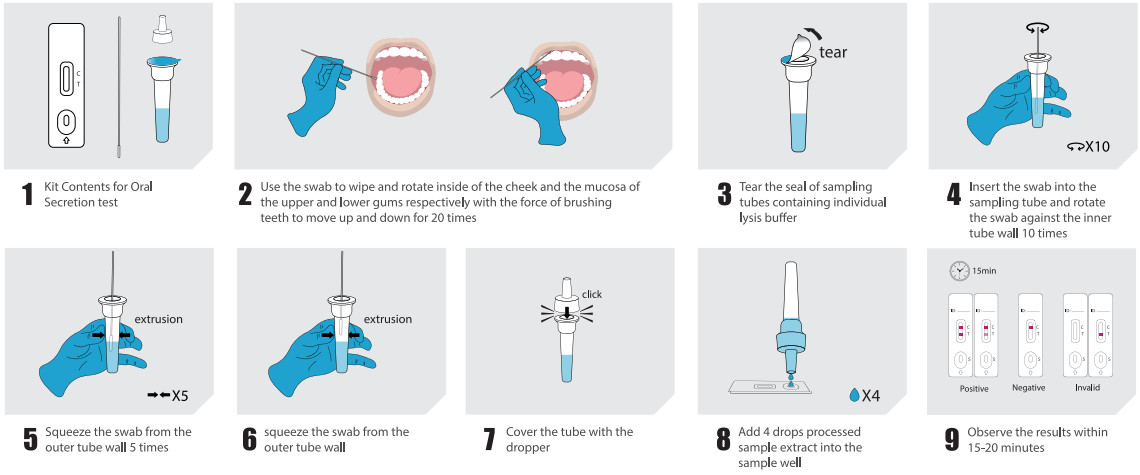
●Oral secretion collection: The sample should be collected in the morning,avoiding food, water or brushing of teeth. Put the swab into the mouth,wipe the inside of the cheek and the mucosa of the upper and lower gums respectively. Use the force of brushing teeth to move up and down, and rotate the swab 20times.
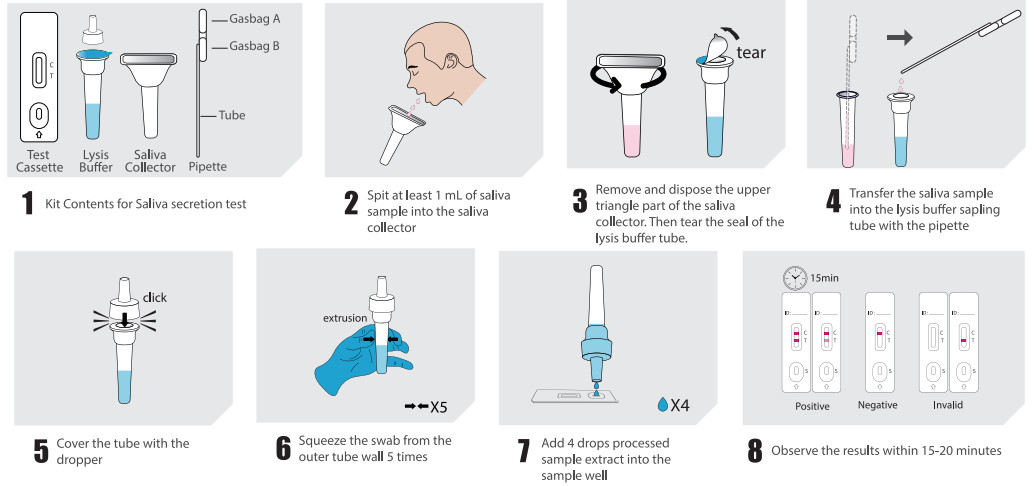
●Saliva secretion collection: The sample should be collected in the morning, avoiding food, water or brushing of teeth until the saliva secretion sample was collected.
Spit at least 1 ml of saliva sample into the saliva collector.
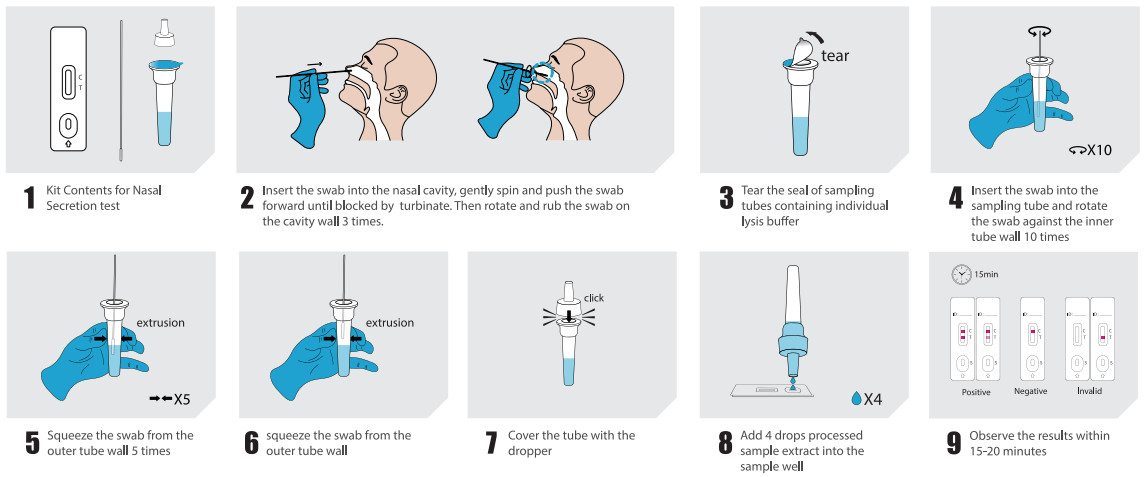
●Nasal secretion collection: Insert the swab into the nasal cavity where there is the most secretion, gently spin and push the swab forward until blocked by the turbinate . Then rotate and rub the swab on the cavity wall 3 times before taking it out.
Sample should be transferred into the lysis buffer provided in this kit as soon as possible after collection.
Test Procedure
Do not open pouch until ready to use. Prep necessary materials: Timer |lAny necessary personal protective equipment.
Type IV | Oral secretion test: Insertthe swab (after cllction) into the sampling tube containing indiviallysis buffer. Rotate the swab against theinner tube wall 10 times and squeeze the swab from the outer tubewall5 times to completely dissolve the sample in the buffer, then move the swab upuntil it is resting on the sample solution, squeeze the swab from the outer tube wall inorder to leave the sample in the tube as much as possible. Remove and discard the swab, cover the tube with the dropper. Open the aluminum foil pouch, takeout the test cassetteand lay it on a clean flat surface, add 4 drops (approximately 100 μL) processed sample extract into the sample well. The result should be observed within 15-20 minutes. Result observed after 20 minutes is invalid.
Type V | Saliva secretion test: After collection, remove and dispose the upper triangle part of the salia collector. Then tear the seal of the lysis buffer tube. Press gasbag Aof the pipette and dip it into saliva sample. Release gasbag A until the sample fldl the whole tube. Then press gasbag A to transfer the sample into the pre-opened lysis buffer. Cover the sampling tube with the dropper and squeeze the tube from the outer wall5 times to completely dissolve the sample in the buffer. Open the aluminum foil pouch, take out the test cassette and lay iton a clean flat surface, add 4 drops (approximately 100μL) processed sample extract into the sample well. The results should be observed within 15- 20 minutes. Result observed after 20 minutes is invalid.
TypeVl |Nasal secretion test:Insert the swab (after cllection) into the sampling tube containing individuallysis buffer Rotate the swab against the inner tube wall 10times and squeeze the swab from the outer tube wall5 times to completely dissolve the sample in the buffer, then move the swab up until it is resting on the sample solution, squeeze the swab from the outer tube wall in order to leave the sample in the tube as much as possible. Remove and discard the swab, cover the tube with the dropper. Open the aluminum foil pouch, take out the test cssette and lay it on a clean flat surface, add 4 drops (approximately 100 μL) processed sample extract into the sample well. The result should be observed within 15-20 minutes. Result observed after 20 minutes is invalid.
Test Method Limitations
●The accuracy of the test is dependent on the quality of the sample. Improper sampling or storage, using expired samples or repeated frozen-thawed samples can affect the test result. Test results can also be affected by temperature and humidity.
●Negative results may be caused by low concentration of SARS-CoV-2 antigens in the sample and therefore cannot completely rule out the possibility of infection.
●Some medication (e.g. high concentration of over-the-counter (OTC) or prescription medication such as nasal spray) in the collected samples may interfere with the test result. Please perform the test again if the result is in doubt.
●This product is only for qualitative testing and the specific concentration of each indicator must be measured using other quantitative methodologies.
●The results of this test are for clinical reference only and should not be the only basis for diagnosis. Results should be used in combination with clinical observations and other testing methods.
Display of Results/Expected Values
●Negative result: If only the quality control C line appears and the detection T line appear is not visible, the sample contains no SARS-CoV-2 antigens or the SARS-CoV-2 antigens concentration is lower than the limit of detection and the result is negative.
●Positive result: If both the quality control C line and the detection T line appear, then the SARS-CoV-2 antigens have been detected and the result is positive.
●Invalid result: If the C line does not appear, the result is invalid and a new test must be performed.
Note: The color intensity of the T line is related to the concentration of SARS-CoV-2 antigens contained in the sample, and the result should be determined by whether
the T line is colored or not regardless of the color intensity.
Product Performance
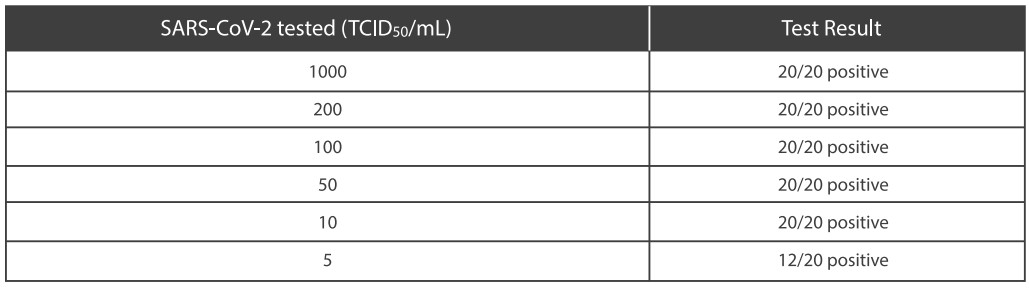
●Limit of Detection-LoD
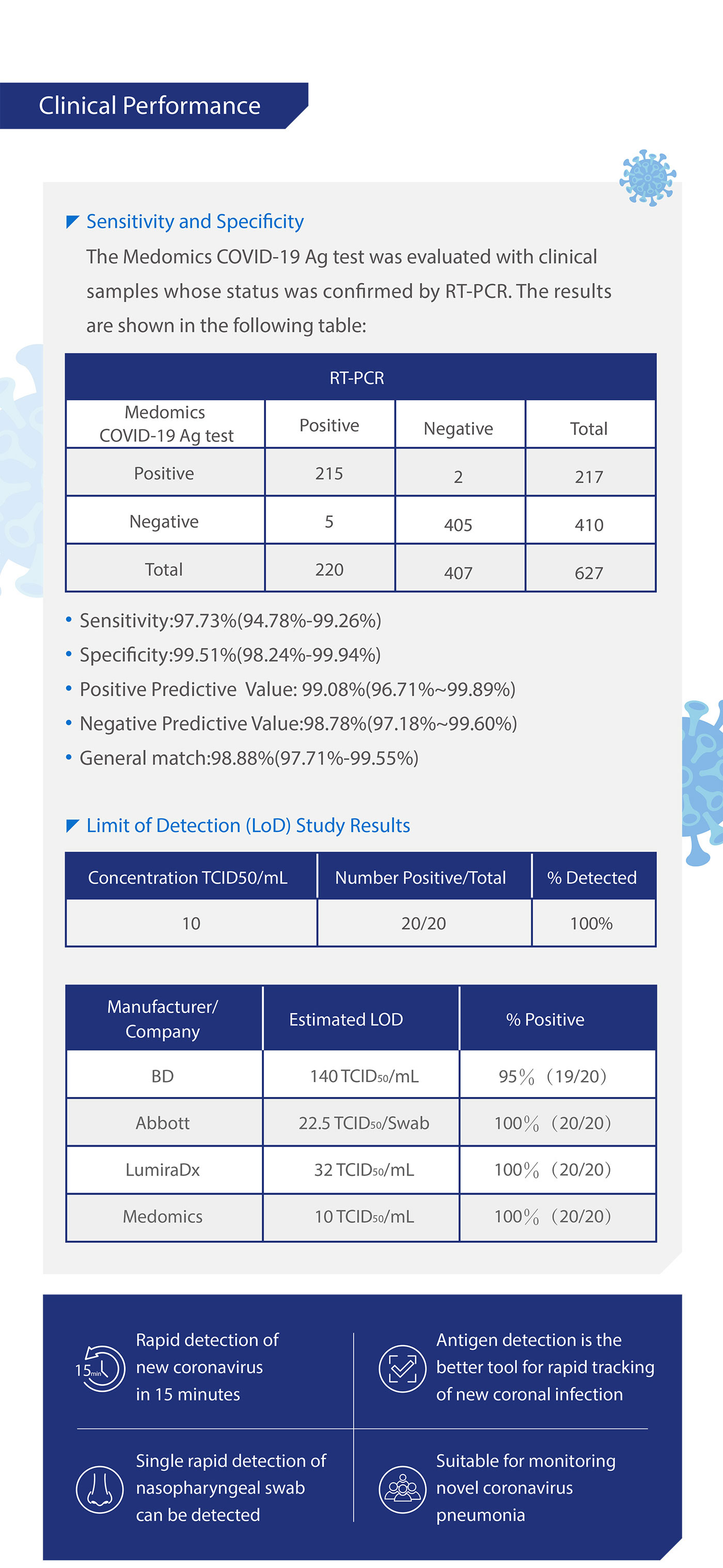
Limit of Detection (LoD) studies determined the lowest detectable concentration of SARS-CoV-2 at which≥95% of all (true positive) replicates test positive. Dilute the SARS-CoV-2 virus with lysis buffer to a final concentration gradient of 5,10, 50, 100, 200, 1000 TCID50/mL.
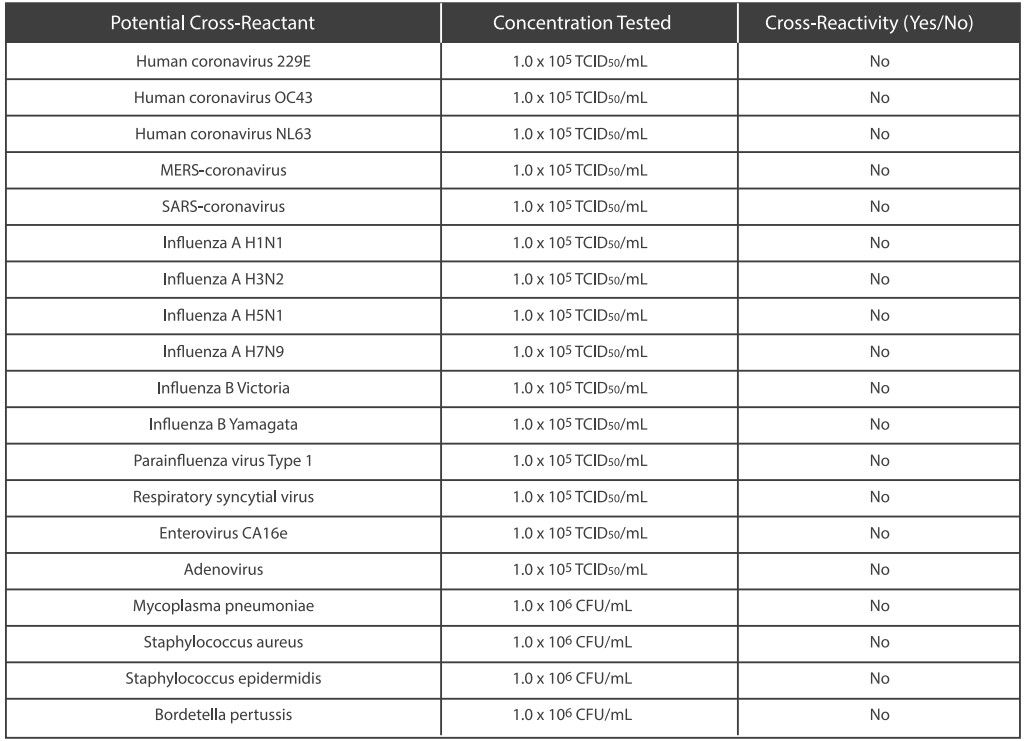
●Cross Reactivity
Cross reactivity and potential interference of Medomics SARS-CoV-2 antigen Test Kit (LFIA) were evaluated by testing commensal and pathogenic microorganisms listed in the following table that may be present in the clinical samples. Each of the bacterium, viruses, and yeast was tested in triplicate with no false positive results.
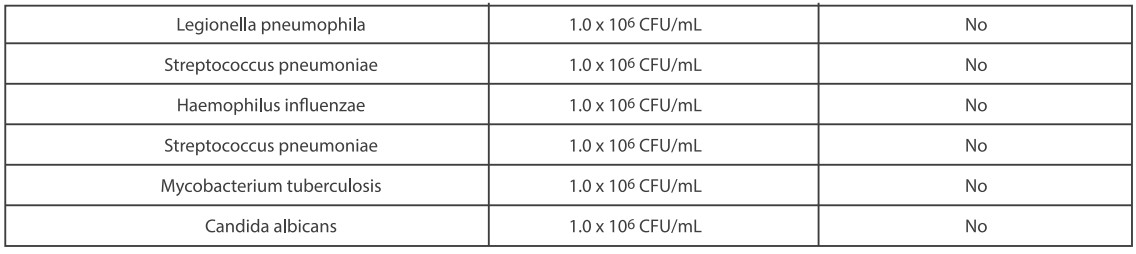
●Endogenous Interfering Substances Effect
A study was performed to evaluate and demonstrate that the endogenous substances naturally present or drugs that may be artifcially introduced into clinical samples do not inference with the detection of SARS-CoV-2 in the Medomics SARS-CoV-2 antigen Test Kit (LFIA) at the concentrations listed below.
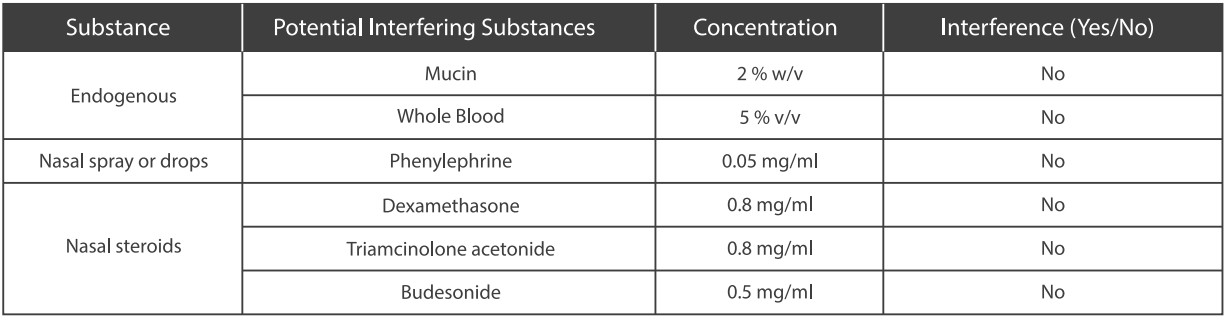
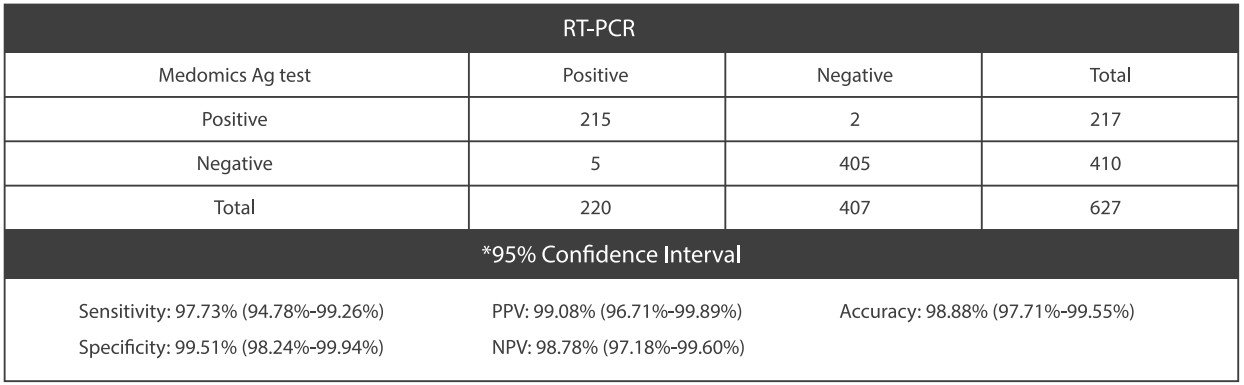
●Clinical Performance
The performance of Medomics SARS-CoV-2 antigen Test Kit (LFIA) was established with 627 nasopharyngeal swabs or throat swabs collected from patients. Two nasopharyngeal swabs were collected from patients and one swab was tested directly using Medomics Ag Test cassette. The real-time Polymerase Chain Reaction (RT-PCR) assay for the detection of SARS-CoV-2 was used as the comparator method to confirm the status of samples for this study.