Introduction
Coronavirus (CoV) belongs to the order Nidovirales under the Coronaviridae family with 4 genera: α, β, γ and δ. The α and β genera are only pathogenic to mammals, while γ and δ genera mainly cause bird infections. CoV is mainly transmitted through direct contact with secretions or through aerosols and droplets. There is also evidence supporting fecal-oral transmission.
7 kinds of human coronaviruses (HCoV) that cause human respiratory diseases have been identified so far, including: HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2. SARS-CoV-2 is one of the most contagious viral pathogens that causes human respiratory tract infections (RTI). Currently, the patients infected by SARS-CoV-2 are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The clinical manifestations include fever, fatigue, cough and other symptoms, accompanied by dyspnea, which can rapidly develop into life-threatening severe pneumonia, respiratory failure, acute respiratory vesicle syndrome, septic shock, multiple organ failure, and severe metabolic acid-base imbalance.
Influenza, usually called flu, is an acute respiratory infection caused by Influenza virus. It is highly contagious. It is mainly spread through coughing and sneezing. It usually breaks out in spring and winter. It is mainly divided into Influenza A and B Influenza virus. Influenza A viruses are highly variable, followed by Influenza B viruses. Therefore, Influenza A viruses are more prevalent and severe, followed by Influenza B viruses. Influenza A includes H1N1, H3N2, H5N1, H7N9, and Influenza B includes Influenza B (Victoria) and Influenza B (Yamagata).
Human adenovirus(ADV) belongs to the adenoviridae family, mammalian adenovirus genus, which is a double-stranded DNA virus without an envelope, mainly infects human respiratory tract, digestive tract and urogenital tract. The main ADV related to respiratory diseases is ADV-B Group (ADV-3, 7, 11, 14, 16, 21, 50, 55), ADV-C Group (ADV-1,2,5,6) and ADV-E group (ADV-4). Acute respiratory adenovirus (ADV) infection which is one of
the most common acute respiratory infections in infants and young children. It mainly causes fever, cough, dyspnea and other symptoms.
Respiratory syncytial virus (RSV) belongs to Pneumovirus of Paramyxoviridae with only one serotype, which is a single stranded negative-strand RNA virus with an envelope. RSV infection mainly causes bronchiolitis and pneumonia in infants under 6 months of age and upper respiratory tract infections such as rhinitis and colds in older children and adults.
Group A streptococci, STREP A, is called streptococcus pyogenes, which belongs to β hemolytic streptococcus. Common mode of transmission is spread through the respiratory tract. STREP A can invade any part of the human body, but upper respiratory tract infection is the most common, followed by skin and soft tissue infection. STREP A can cause both suppurative diseases and non- suppurative complications. STREP A is also an indirect cause of the allergic diseases rheumatic fever and acute glomerulonephritis.
Intended Use
SARS-CoV-2/FluA/FluB+ADV/RSV/SA Antigen Combo Rapid Test Kit (LFIA) is an immunochromatography based one step in vitro test. It is designed for the rapid qualitative determination of SARS-CoV-2, Influenza A , Influenza B virus, Adenovirus(ADV), Respiratory syncytial virus(RSV) and Group A streptococci (STREP A) antigen in swab samples from individuals suspected of COVID-19, Influenza A , Influenza B, Adenovirus(ADV), Respiratory syncytial virus(RSV) and Group A streptococci (STREP A). It can be used for detecting SARS-CoV-2, Influenza A , Influenza B virus, Adenovirus(ADV), Respiratory syncytial virus(RSV) and Group A streptococci (STREP A). Which is often used as an auxiliary method in the clinical diagnosis, but not as the only basis.
Test Principle
SARS-CoV-2/FluA/FluB+ADV/RSV/SA Antigen Combo Rapid Test Kit (LFIA) uses a double antibody sandwich method to detect SARS-CoV-2 ,Influenza A,influenza B, Influenza B virus,Adenovirus(ADV), Respiratory syncytial virus(RSV) and Group A streptococci (STREP A) by colloidal gold immunochro matography. When the appropriate amount of test samples treated with lysis buffer is added to the sample well of the test cassette, the sample will
move forward along the test strip by capillary action. If the sample contains SARS-CoV-2,Influenza A/B virus, Adenovirus(ADV), Respiratory syncytial virus(RSV) and Group A streptococci (STREP A) antigen, and the concentration is higher than the limit of detection, the antigen will form immune complexes with corresponding Nucleocapsid Protein antibody labeled with colloidal gold respectively, which are captured by lines NCoV line, Flu A line, Flu B line,ASV line,RSV line and SA line. If test sample contains SARS-CoV-2 virus, forming a red NCoV line, indicating a positive result for SARS-CoV-2. If test sample contains Influenza A virus, forming a red Flu A line, indicating a positive result for Influenza A, If test sample contains Influenza B virus, forming a red FluB line, indicating a positive result for Influenza B.If test sample contains Adenovirus, forming a red ASV line, indicating a positive result for Adenovirus. If test sample contains Respiratory syncytial virus, forming a red RSV line,indicating a positive result for Respiratory syncytial virus. If test sample contains Group A streptococci, forming a red SA line, indicating a positive result for Group A streptococci.
Additionally, the test strip also contains a control line (C line). The C line should be formed to indicate that the sample has been transported properly through the membrane regardless of whether sample contains antigens or not. If the C line does not appear, it indicates that the test result is invalid and the sample need to retest.
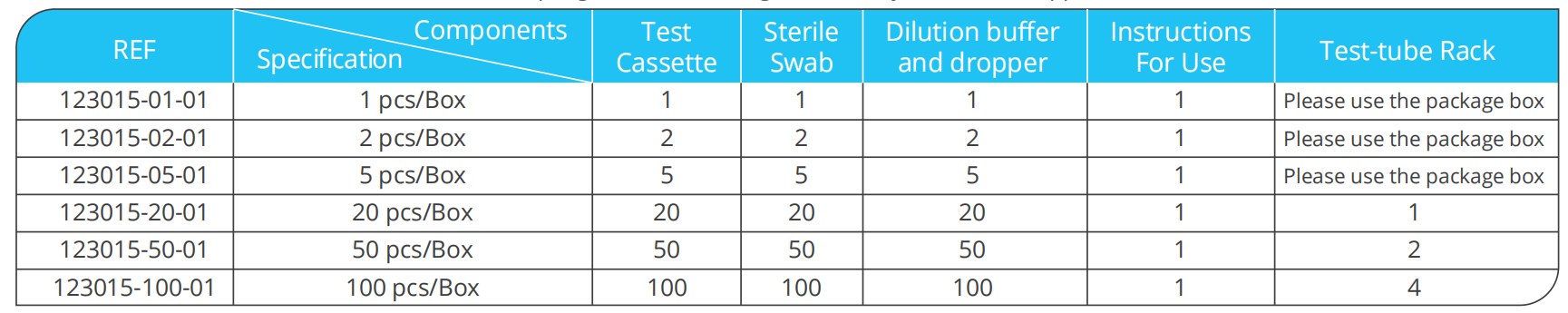
Test Kit Contents
Test kit contains test cassettes, sterile swabs, sampling tubes containing individual lysis buffer, droppers and instructions for use.
Test cassette: contains the SARS-CoV-2 & Influenza A/B test strip , ADV, RSV and STREP A Antigen test strip and a plastic cassette casing. SARS-CoV-2 & Influenza A/B Antigen test strip contains anti-SARS-CoV-2 Nucleocapsid Protein antibody labeled with colloidal gold, anti-Influenza A Nucleocapsid Protein antibody labeled with colloidal gold, anti-Influenza B Nucleocapsid Protein antibody labeled with colloidal gold. Another anti-SARS-CoV-2
Nucleocapsid Protein antibody, anti-Influenza A Nucleocapsid Protein antibody and anti-Influenza B Nucleocapsid Protein antibody are fifixed on the NCoV line, Flu A line and Flu B line respectively. The NCoV line/Flu A line/FLu B line and control line (C line) are in the detection window on the nitrocellulose membrane.
ADV, RSV and STREP A Antigen test strip contains anti-ADV antibody labeled with colloidal gold, anti-RSV antibody labeled with colloidal gold, anti-STREP A antibody labeled with colloidal gold. Another anti-ADV antibody, anti-RSV antibody and anti-STREP A antibody are fixed on the ADV line, RSV line and SA line respectively. The ADV line/RSV line/SA line and control line (C line) are in the detection window on the nitrocellulose membrane.
Test Method Limitations
1.The accuracy of the test is dependent on the quality of the sample. Improper sampling or storage, using expired samples or repeated frozen-thawed samples can affect the test results. Test results can also be affected by temperature and humidity.
2.Negative results may be caused by low concentration of SARS-CoV-2, Influenza A, Influenza B, ADV, RSV, STREP A antigens in the sample and therefore cannot completely rule out the possibility of infection.
3.Some medication (e.g. high concentration of over-the-counter (OTC) or prescription medication such as nasal spray) in the collected samples may interfere with the test result. Please perform the test again if the result is in doubt.
4.This product is only for qualitative testing and the specific concentration of each indicator must be measured using other quantitative methodologies.
5.The test results of this kit are for clinical reference only and are not the sole basis for clinical diagnosis. The clinical diagnosis and treatment of patients should be comprehensively considered in combination with their symptoms/signs, medical history, other laboratory tests and treatment response.
Product Performance
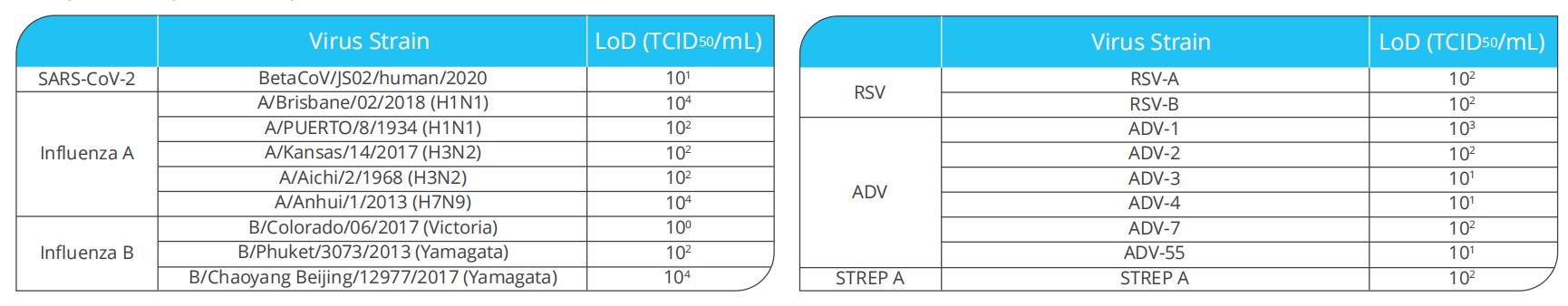
• Limit of Detection-LoD
Limit of Detection (LoD) studies determined the lowest detectable concentration of SARS-CoV-2, Influenza A, Influenza B , ADV, RSV and STREP A at which 100% of all (true positive) replicates test positive.
• Cross Reactivity
Cross reactivity of SARS-CoV-2/FluA/FluB+ADV/RSV/SA Antigen Combo Rapid Test Kit (LFIA) was evaluated by testing commensal and pathogenic microorganisms listed in the following table that may be present in the clinical samples. Each of the bacterium, viruses, and yeast were tested in triplicate with no false positive results of SARS-CoV-2 virus, Influenza A , Influenza B, ADV, RSV and STREP A.