A Rapid lgM-IgG Combined Antibody Test Kit for SARS-CoV-2 (ICA)
Instructions For Use
Intended Use
The Medomics Rapid lgM-IgG Combined Antibody Test for SARS-CoV-2 is a lateral flow immunoassay intended for the qualitative, differential detection of lgG and lgM antibodies to SARS-CoV-2 in human serum, plasma, or whole blood samples (capillary or venous) including samples prepared by commonly-used anticoagulants (K2EDTA, NaCitrate, Li-Heparin) from individuals with current or prior COVID-19 infection. The Medomics Rapid lgM-lgG
Combined Antibody Test for SARS-CoV-2 is intended for use as an aid in identifying patients with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Medomics Rapid lgM-IgG Combined Antibody Test for SARS- CoV-2 should not be used to diagnose acute SARS-CoV-2 infection.
Results are for the detection of SARS CoV-2 antibodies. lgM and lgG antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although the duration of time antibodies are present post-infection is not well characterized. Patients may have detectable virus present for several weeks following seroconversion.
The sensitivity of Medomics Rapid lgM-lgG Combined Antibody Test for SARS-CoV-2 early after infection is unknown. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
False positive results for Medomics Rapid lgM-lgG Combined Antibody Test for SARS-CoV-2 may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using a second, different IgG or IgM assay.
Summary
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by novel coronavirus ; are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation periodis 1 to 14
days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Test Principle
Medomics Rapid lgM-lgG Combined Antibody Test for SARS- :CoV-2 is immunochromatography based. The test card contains (1) colloidal gold-labeled recombinant novel coronavirus antigen, (2) two detection lines (G and M lines) and one quality control line (C) fixed on a nitrocellulose membrane. M is fixed with monoclonal anti-human lgM antibody for detecting the novel coronavirus IgM antibody. G is fixed with monoclonal anti-human lgG antibody for detecting the novel coronavirus lgG antibody. The quality control antibody is fixed on the C line.
When an appropriate amount of test sample is added to the sample well of the test cassette, the sample will move forward along the test card via capillary action. If the sample contains lgM antibody, the antibody will bind to the colloidal gold- labeled novel coronavirus antigen.
The antibody/antigen complex will be captured by the anti-human lgM antibody immobilized on the membrane, forming a red M line and indicating a positive result for the lgM antibody.
If the sample contains lgG antibodies, the antibody will bind to the colloidal gold-labeled novel coronavirus antigen and the antibody/antigen complex will be captured by the antibody immobilized on the membrane, forming a red G line and indicating a positive result for the IgG antibody.
If neither antibody is present, a negative result is displayed. The card also contains a quality control line (C). Regardless of what antibodies are present the C line should appear to indicate that the sample has been transported properly through the membrane. If the C line does not appear it indicates that the test result is invalid and a new, unopened test cassette is required to repeat the test.
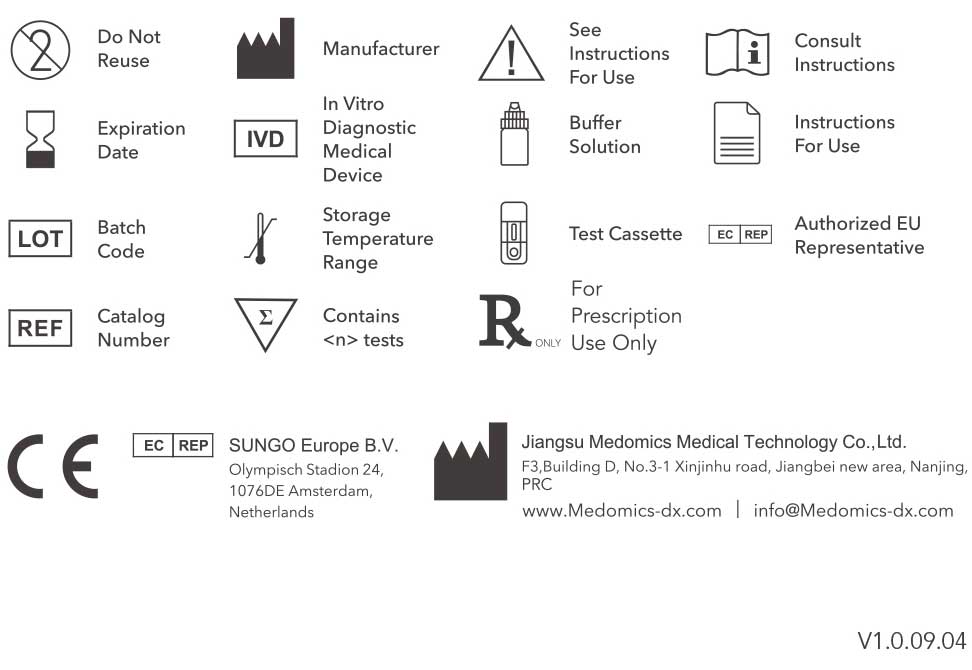
Contents of the Kit
One test kit contains:
20 Test Cassettes | 1 Buffer Solution Bottle | 1 Package Insert
One test cassette contains:
●Dried reagents with stabilizers
●Colloidal gold-labeled novel coronoavirus antigen
●Antimouse lgG polyclonal antibody
●Anti-human lgG monoclonal antibody
●Anti-human lgM monoclonal antibody
Materials not provided but required:
Sampling Devices | Alcohol Wipes | Gloves| Timer
Warnings and Precautions
●For human in vitro clinical diagnostics only.
●The product should only be used by trained healthcare professionals.
●After opening the sealed cassette pouch the test should be used within one hour.
●Do not immerse test cassette in water.
●Do not freeze test cassette or buffer solution.
●Handle specimens in accordance to the OSHA Standard on Bloodborne Pathogens.
●Wear protective gloves, clothing, and eyewear.
●Wash hands thoroughly after handling specimens.
●Dispose of all used or damaged test cassettes, capillary samplers, or other kit components as biohazardous materials.
●Do not use test cassette, buffer solution, or any other kit components if the pouch is damaged or the seal is broken.
●Do not use samples containing lipids, hemolysis, or turbidity which can affect results.
●Not for use with heat inactivated or other inactivated human specimen (blood, serum,plasma).
●This package insert must be read completely before performing the test. Failure to follow directions in insert may yield inaccurate test results.
●Test results should be read between 10 and 15 minutes after a specimen is applied to the sample well. Results read after 15 minutes may give erroneous results
●Do not use test cassette, buffer solution, or any kit component beyond the indicated expiration date.
●Bring all reagents to room temperature (15-30°C) before use.
●Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
●Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
Storage Instructions
The reagent should be stored in the dark at room temperature (2°C to 30°C) and has a shelf-life of 12 months. The container should be protected from light after being opened.
Do not freeze.
Sample Requirements
●Suitable for human serum, plasma, or whole blood samples (capillary or venous) including samples prepared by commonly-used anticoagulants (K2EDTA, NaCitrate, Li- Heparin).
●Fresh samples should be collected and tested without inactivation.
●Not for use with heat inactivated or other inactivated human specimen (blood, serum, plasma).
●Serum and plasma samples can be stored at 2-8°C for 5 days. If long-term storage of serum or plasma samples is required, store at -20°C and avoid repeated freeze/thaw cycles.
●Anticoagulated whole blood samples can be stored at 2-8°C for 5 days.
●Before testing, samples stored in refrigerated or frozen storage should be slowly returned to room temperature (15-30° C) and stirred. When particulates are clearly visible in the sample the precipitate should be removed by centrifugation before testing.
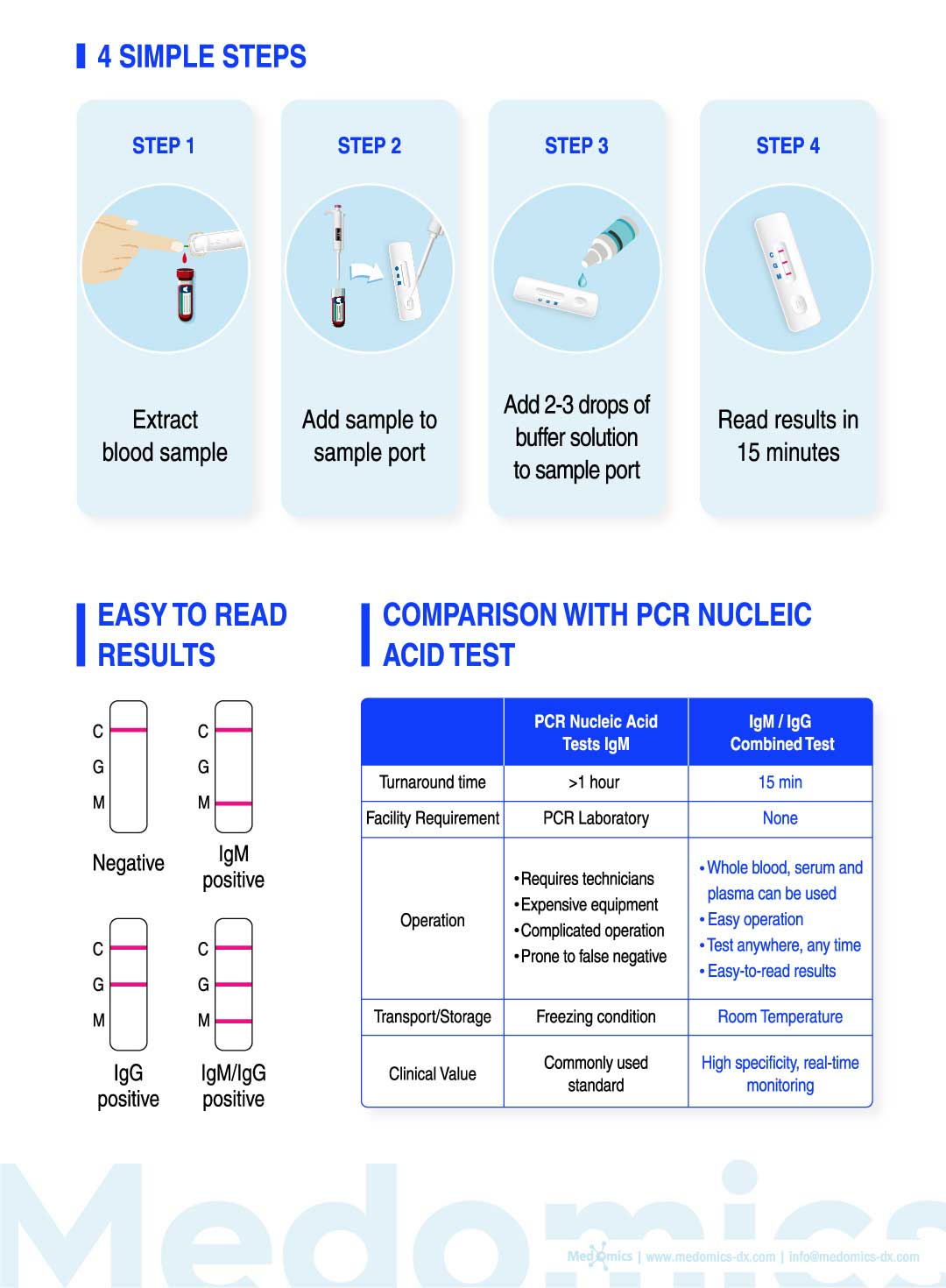
Test Procedure
Do not open pouch until ready to use. Prep necessary materials: Test cassette| Buffer
solution | Sampling Device Label Test cassette with patient ID.
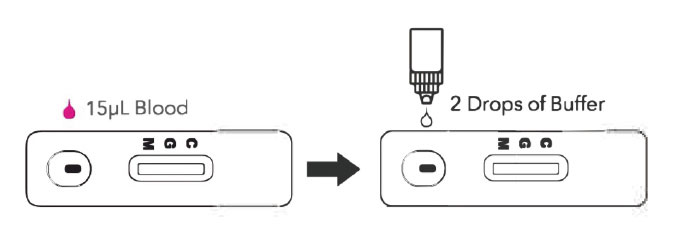
1 | Obtain a specimen using standard laboratory or provider protocols. Using appropriate
sampling device, obtain 15μL of fingerstick or venous whole blood specimen, or 10μL of
serum or plasma using appropriate titration device.
●For intravenous sampling follow standard laboratory protocols.
2 | Dispense the specimen into the Test Cassette sample well.
●Ensure that the entire sample is dispensed into the sample well.
3 | Remove colored cap of the Buffer Solution bottle and dispense 2 drops into the Test
Cassette sample well.
●Remove any air bubbles in the dropper.
●Test on a level surface at room temperature.
4 | Allow test to run for 10 minutes. Read the results by viewing the detection window.
●Test results that have run over 15 minutes are invalid.
Test Method Limitations
●This product can only be used to detect the lgG and IgM antibodies of the novel coronavirus in human whole blood (capillary or venous), serum, or plasma. It cannot be used with other body fluids or secretions.
●Proper sample collection is critical for optimum test performance. Failure to follow the collection and sampling requirements may give inaccurate results.
●This product is only for qualitative testing and the intensity of the test line does not necessarily correlate to SARS-CoV-2 antibody titer in the specimen.
●Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
●Negative results may be caused by low concentrations of the novel coronavirus lgG/lgM antibody in the sample and therefore cannot completely rule out the possibility of infection.
●Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. Results should be used in combination with clinical observations and other testing methods.
●Test results can be affected by temperature and humidity.
●A negative or non-reactive result can occur if the virus has undergone minor amino acid mutation(s) in the epitoperecognized by the antibody detected by the test.
●This test should not be used for screening of donated blood.
Display of Results/Expected Values
A total of three detection lines are possible, with the control (C) line appearing when sample has flowed through the cassette.
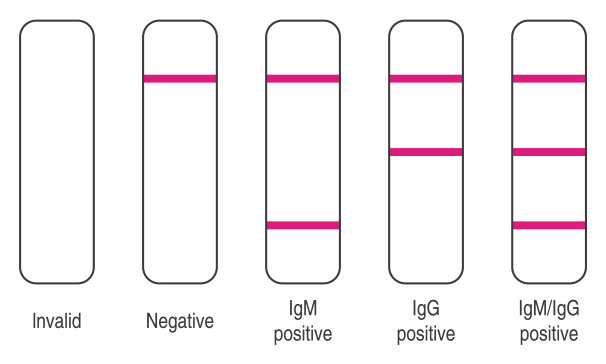
1 | Invalid Result: If the quality control line (C) does not appear, then the test result is invalid and sample must be retested with a new cassette.
2 | Negative Result: If only the quality control line (C) appears and the detection lines G and M are not visible, then no novel coronavirus antibody has been detected and the result is negative.
3 | Positive Result, M only: If both the quality control line (C) and the detection line M appears, then the novel coronavirus lgM antibody has been detected and the result is positive for the IgM antibody.
4| Positive Result, G only: If both the quality control line (C) and the detection line G appears, then the novel coronavirus lgG antibody has been detected and the result is positive for the lgG antibody.
5 | Positive Result, G and M: If the quality control line (C) and both detection lines G and M appear, then the novel coronavirus IgG and lgM antibodies have been detected and the result is positive for both the lgG and IgM antibodies.
Internal Quality Control Procedure
Each Test Cassette device has a built-in control. A red colored line in the detection window at the Control line can be considered an internal positive procedural control. The Control line will appear if the test procedure has been correctly performed. If the Control line does not appear, the test is invalid and a new test must be performed. If the problem persists, please contact your local vendor or Medomics for technical support.
Performance Characteristics
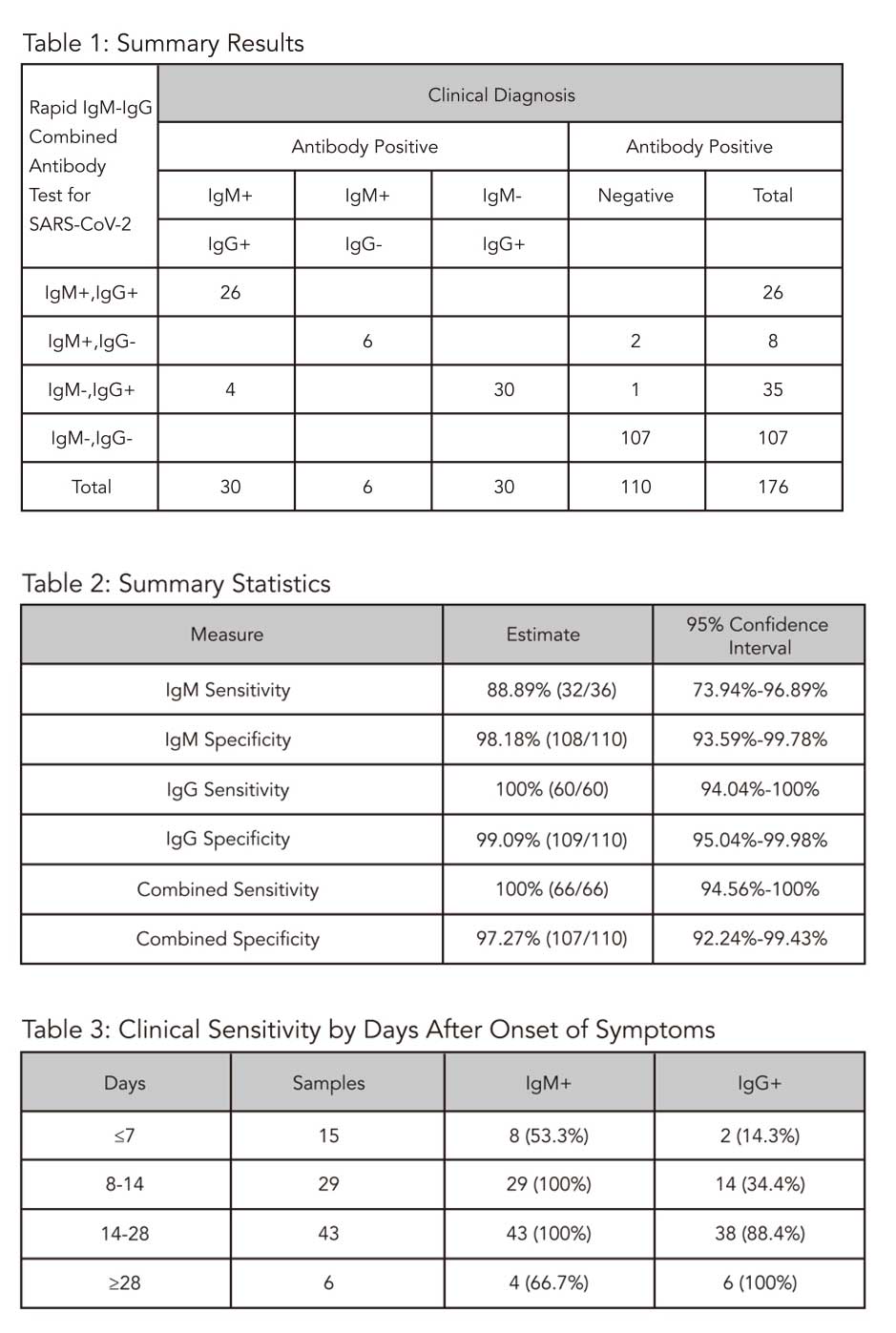
Clinical Agreement Validation Study| The Medomics Rapid lgM- lgG Combined Antibody Test for SARS-CoV-2 was self-validated using clinical samples. The test was validated against a panel of previously frozen samples consisting of sixty-six (66) SARS-CoV-2 antibody-positive and one hundred and ten (1 10) antibody- negative plasma samples. The antibody-positive samples were collected from clinically confirmed COVID- 19 infected patients confirmed with BioSciences chemiluminescent lgM and IgG assay (magnetic particles) which was approved by The National Medical Products Administration (NMPA).
Within the 66 positive samples, there were 30 samples with both lgM and IgG antibodies present, 6 samples with lgM only present and 30 samples with lgG only present. All 110 negative specimens were collected from healthy donors and confirmed SARS-CoV-2 antibody negative with BioSciences chemiluminescent lgM and lgG assay.
Testing was performed using one lot of the Medomics Rapid lgM-lgG Combined Antibody Test for SARS-CoV-2. Confidence intervals for SARS-CoV-2 displayed a combined sensitity of 100% and a combined specificity of 97.27%.
Cross Reactivity | A cross-reactivity study was performed using forty-four commercially obtained sera specimens (serum or sodium citrate plasma). The full specimen list and donor demographic information can be found in the study report.
All specimens were associated with a COVID-19 respiratory negative PCR result or were collected prior to the COVID-19 outbreak. Each specimen was tested in duplicate for a total of 88 sample results. Three blinded readers independently read each cassette. Results were collated at the end of the study and adjudicated based on agreement of two of three readers. Cassettes were also read by a Hamamatsu reflectance reader.
The protocol followed the established instructions for use. For each test 10 μl of sample was applied to the cassette, allowed to sit for 10 minutes before interpretation. Cross-Reactivity Results for the Medomics Rapid lgM-IgG Combined Antibody Test for sensitivity and specificity were calculated per a score method described in CLSI EP12-A2 (2008). The results and data analysis are shown in the tables below.The Medomics Rapid lgM-lgG Combined Antibody Test for SARS-CoV-2 are shown in the table below.
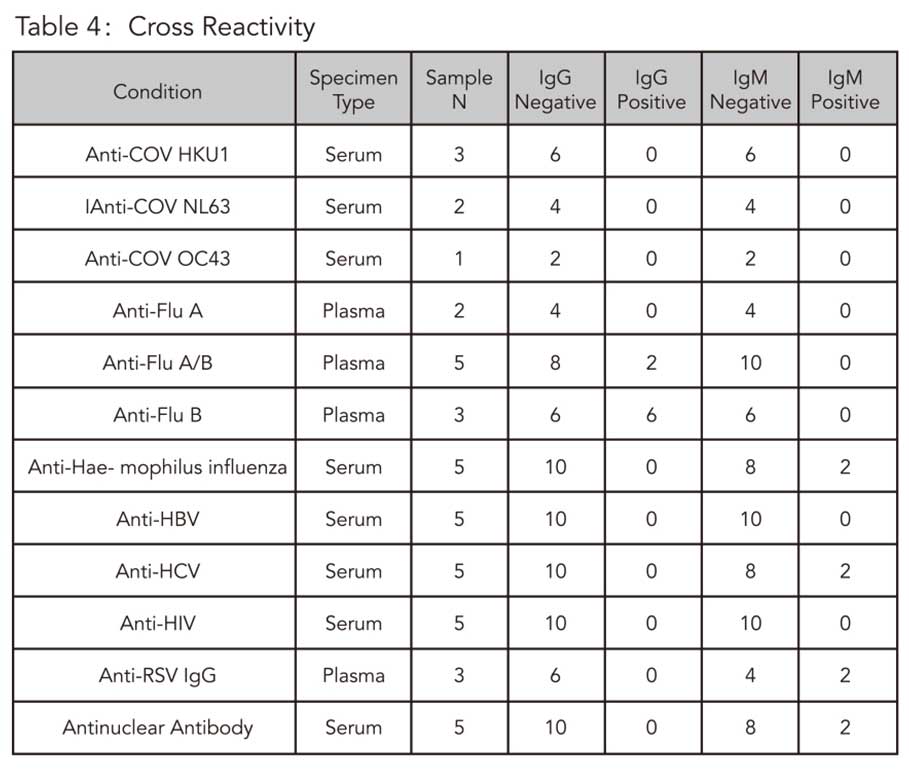
The positive lgM and lgG bands for all replicates except Anti- HCV (lgM) were observed as faint and therefore not considered significant interference. However, false positive results due to cross-reactivity with antibodies to anti-HCV could occur