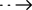PRINCIPLE
This kit uses an immunofluorescence double antibody sandwich assay to quantify the concentra�tion of cardiac troponin (cTnI)/myoglobin (Myo)/creatine kinase (CK-MB) in human whole blood, plasma and serum samples. After the diluted samples are added dropwise to the sample wells of the assay kit, cTnI in the samples binds to the rare-earth fluorescently labeled anti-cTnI
monoclonal antibody I to form an immunoreactive complex, which moves forward along the nitrocellulose membrane and is captured by the anti-cTnI monoclonal antibody II in the detection zone of the nitrocellulose membrane. Myo in the sample binds to the rare-earth fluorescently labeled anti-Myo monoclonal antibody I to form an immunoreactive complex The complex is captured by the anti-Myo monoclonal antibody II in the detection zone of the nitrocellulose membrane, creating a detection signal. CK-MB in the sample binds to rare-earth fluorescence-labeled anti-CK-MB monoclonal antibody I to form an immunoreactive complex, which moves forward along the nitrocellulose membrane and is captured by anti-CK-MB monoclonal
antibody II in the detection zone of the nitrocellulose membrane, resulting in a detection signal.
The complex moves along the nitrocellulose membrane and is captured by the CK-MB monoclonal antibody II in the detection zone of the nitrocellulose membrane, resulting in a detection signal. The rare-earth fluorescence-labeled antibody that is not captured in the detection zone moves forward along the nitrocellulose membrane and binds to the quality control antibody in
the quality control zone, forming a quality control signal.
The working principle of the supporting instrument: insert the reacted detection card into the Supporting Instrument, the measurement system of the instrument will automatically scan the detection signal area and the quality control signal area to obtain the optical signal, and then measure and analyze the optical signal , quantify the concentration of cTnI+Myo+CK-MB Combo Rapid Test Kit in the tested sample.
MAIN COMPONENTS
The kit contains the corresponding number of test cassettes for each package specification: the test cassette consists of a test strip shell and a test strip, which consists of a binding pad containing fluorescent microsphere-labelled anti-cTnI monoclonal antibody, anti-Myo monoclonal antibody, anti-CK-MB monoclonal antibody and chicken IgY, a sample pad encapsulated with a
C-line quality control antibody, anti-cTnI monoclonal antibody, anti-Myo monoclonal antibody, anti-CK-MB monoclonal antibody, a nitrocellulose membrane, an absorbent paper and a PVC plate. antibodies, nitrocellulose membrane, sample pad, absorbent paper and PVC plate.
Diluent: Phosphate (20 mM PB buffer), preservative (0.05% PC300), surfactant (0.5% Trilatone X-100), pH=7.4±0.2
MATERIAL REQUIRED BUT NOT PROVIDED
Timer, pipette and disposable gun head.
APPLICABLE INSTUMENT
1.MKF-101 fluorescent immunoanalyzer
2.MKF-201 Portable dry fluorescence immunoassay analyzer
3.MKF-202 fluorescent immunoanalyzer
SAMPLE REQUIREMENTS
The Sample types with test cassette include human serum, plasma or whole blood samples Whole blood collection: Blood is collected using sodium heparin, EDTA-K2 or sodium citrate anticoagulation tubes, and the collected blood sample is added and shaken well and set aside.
Samples should not be frozen and should be tested as soon as possible after collection. If the test cannot be completed within 12 hours, it should be stored at 2°C to 8°C for no more than 2 days.
Serum or Plasma collection: Serum and plasma should be separated as soon as possible after blood collection to avoid haemolysis. The serum and plasma should be tested as soon as possible after separation, and if the test cannot be completed within 12 hours, stored at 2-8°C for no more than 7 days. Long-term storage should be frozen at -20°C for no more than 6
months. Samples should not be frozen and thawed more than 2 times. Samples must be returned to room temperature prior to testing. Frozen samples must be completely thawed, mixed and brought back to room temperature before use, and must not be
repeatedly frozen and thawed.