SARS-CoV-2 Neutralizing Antibody Test Kit (ELISA) Instructions For Use
Intended use
The SARS-CoV-2 Neutralizing antibody Test Kit(ELISA) is suitable for invitro qualitative detection of SARS-CoV-2 neutralizing antibodies in humanserum samples.
SARS-CoV-2 Neutralizing antibody is an important marker for assessingthe effectiveness of the new coronavirus vaccines.This reagent is suitablefor neutralizing antibody detection in samples from individuals with vaccineinjection people and recovered people.
SARS-CoV-2 Neutralizing antibody Test Kit(ELISA) is of great significancefor the development of new coronavirus vaccines, evaluation of effective-ness, and evaluation of neutralizing antibody levels in the population.
Summary
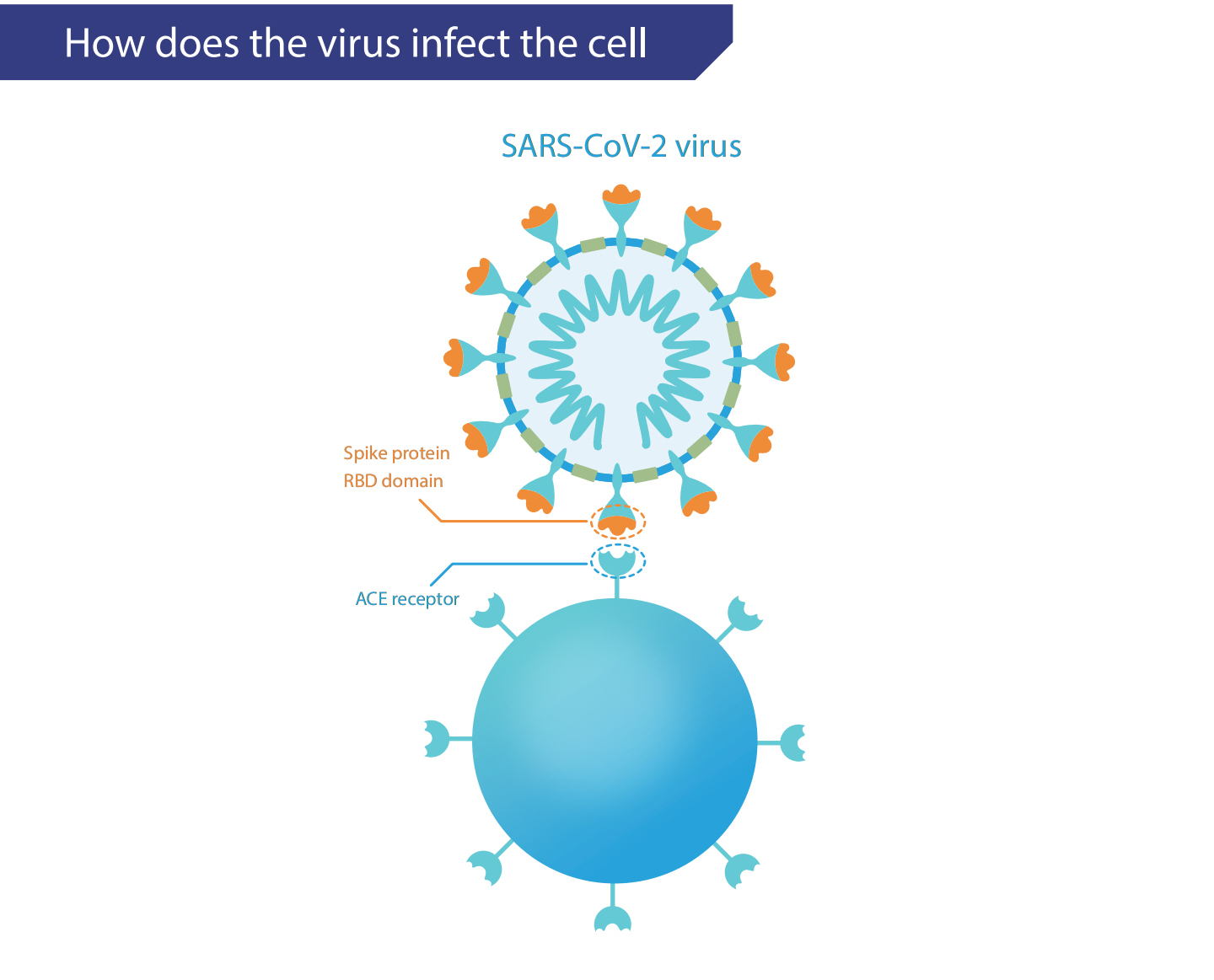
With the global pandemic of the novel coronavirus disease (COVID-19),the number of infections and deaths continues to rise, and scientists fromvarious countries are constantly looking for treatments for COVID-19. Forthe prevention of sudden major infectious diseases, neutralizing antibodytherapy is an important strategy for effective prevention and treatment.Neutralizing antibodies are a kind of soluble protein secreted by adaptiveimmune response cells, it can recognize the virus surface protein andprevent it from binding to cell receptors. After the virus invades the human body, immune cells secrete neutralizing proteins into the blood.These antbodies prevent the virus from infecting cells by binding to thespike protein on the surface of the virus. Neutralizing Antibodies are expected to become a sharp edge against the novel coronavirus disease.
Test Principle
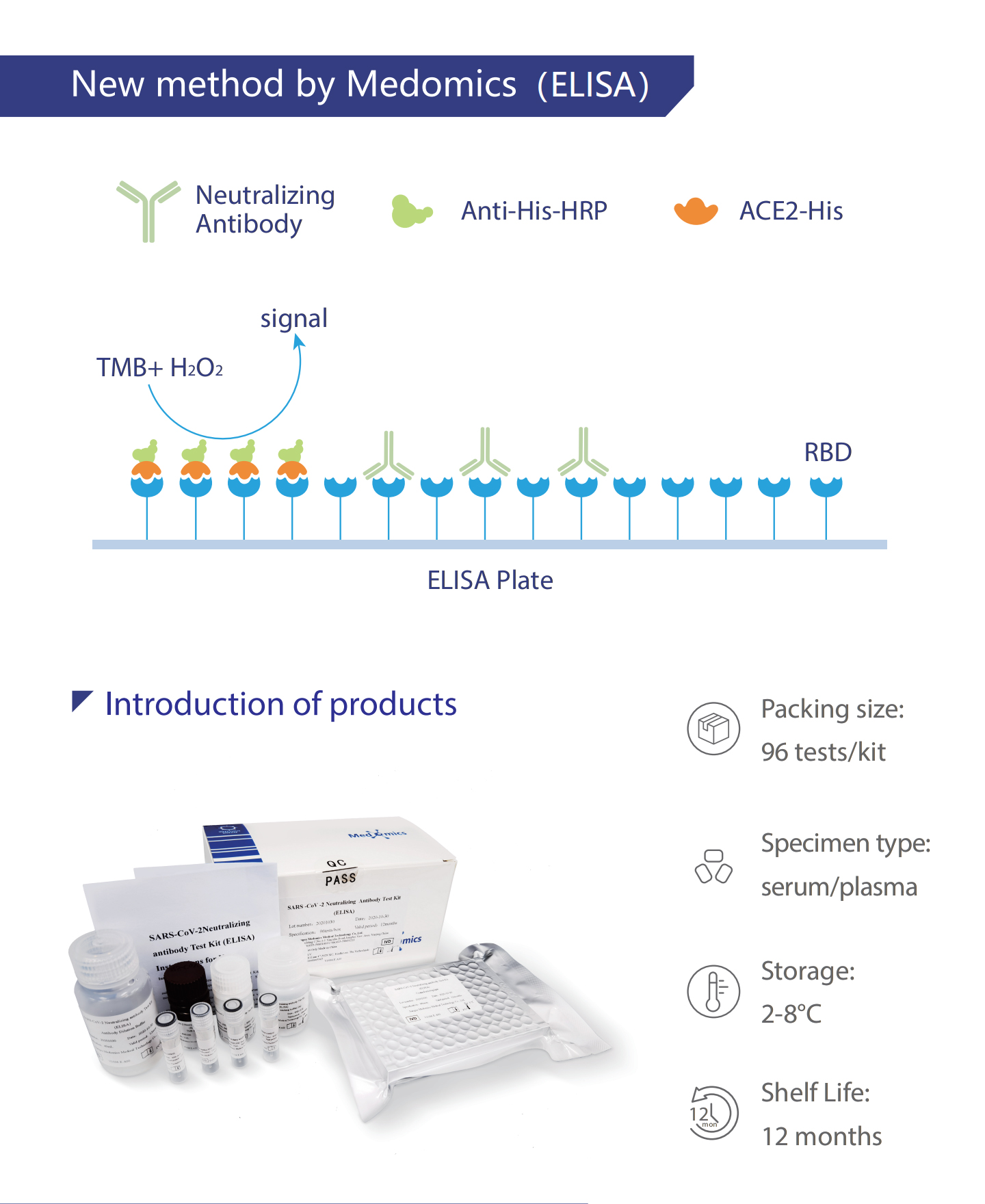
Enzyme-linked immunoassay(El ISA) is used to qualitatively detectwhether the sample contains human anti-viral neutralizing antibodies.
Coat the microplate with recombinant protein RBD, and then add the
samples and ACE2 to incubate. ACE2 competes with the human anti-viralneutralizing antibody in the samples to combine to RBD, and then add thesecondary antibody-HRP against ACE2 antibody to form antigen-anti-
body-secondary antibody-HRP complex, which makes the substrateshow color. The absorbance (OD value) is measured with a microplate reader at a wavelength of 450 nm to calculate the inhibition rate. When thesample inhibition rate value is less than the critical inhibition rate, it is regarded as a negative sample; when the sample inhibition rate is greaterthan or equal to the critical inhibition rate, it is regarded as a positive sample.
Applicable Instrument
Microplate reader
Contents of the Kit
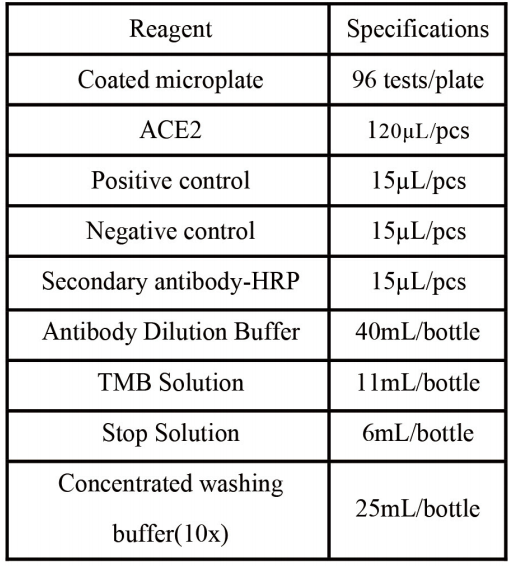
Desiccant, packaging box and instructions for use.
Note: ltems needed for the experiment (not provided) include microplatesealers.
Warnings and Precautions
● Read the instructions carefully before use.
●The reagent must not be in contact with skin or mucous membranes. If thereagent comes in contact with skin, immediately rinse with plenty ofwaterand ask medical professionals for treatment
● The reagent should be used within its specified shelf life.
● For human in vitro clinical diagnosis only.·Test results are for clinical reference only.
●There may be differences in testing the same sample using reagents fromdifferent manufacturers.
●Samples: All samples and reaction waste should be treated as the sourceof infection, and the operators should take necessary protective measures.
Storage Instructions
The reagent should be stored in dark at 2-8C with a shelf-life of 12 months.
Sample Requirements
Suitable for human serum samples.
●After the sample is collected, it should be tested immediately. Serumsamples can be stored at 2-8C for 5 days.
●Before testing, slowly return the sample to room temperature and mix well.If there are obvious visible particulate matter or floccules in the sample,remove it before test.
● Do not use samples containing lipids, hemolysis, or turbidity which canaffect results.
●The sample should not contain NaN3.When the RF concentration in thesample is less than or equal to 400IU/mL, the HAMA concentration is lessthan or equal to 100ng/mL, the bilirubin concentration is less than or equalto 18umol/L, the hemoglobin concentration is less than or equal to 100g/L,and the triglyceride concentration is less than or equal to 20mmol/L ,the substances are higher than the above concentrations, the accuracy ofthe results may be affected. In this case, this method is not applicable.
Test Procedure
1. Reagent preparation:
1.1 The negative control, positive control, and samples arerecommended to be diluted with antibody dilution buffer at a ratio of1:10.
1.2 ACE2 working buffer: Diute the ACE2 with antibody dilution bufferat a ratio of 1:100.
1.3 Secondary antibody-HRP working solution: Dilute secondaryantibody-HRP with antibody dilution buffer at a ratio of 1:1000.
1.4 Washing buffer: Dilute Concentrated washing buffer(10x) withpurified water at a ratio of 1:10.
2. Test steps:
2.1 Equilibrate the kit to room temperature before use and brieflycentrifuge the reagent tube before opening the lid.
2.2 Addition of samples:
Add 100uL diluted negative control, positive control and samples tothe corresponding wells on the coated microplate; And then add 100pL ACE2 working buffer to each well.
2.3 Incubation: 25C for 30 min.
2.4 Washing: Wash each well 3 times with washing buffer (300uLwell).
2.5 Secondary antibody-HRP:Add 100pL of Secondaryantibody-HRP working solution to each well.
2.6 Incubation: 25C for 30 min.
2.7 Washing: Wash each well 3 times with washing buffer (300uLwell).
2.8 Substrate Reaction: Add 100uL of TMB solution to each well andincubate the plate in dark at 37"C for 10 minutes.
2.9 Stop Reaction: Add 50pL of stop solution to each well to quench thereaction. .
2.10 Reading results: Read the absorbance in the microtiter plate readerat 450 nm as soon as possible or within 15 minutes after adding the stopsolution.
Interpretation of Results
1. Negative quality control OD value > 1.5, positive quality control ODvalue < 1.0; Otherwise, the test results are invalid.
2. Result:
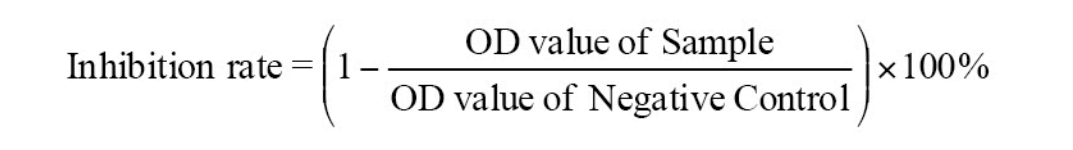 Critical inhibition rate =15%
Critical inhibition rate =15%
When the sample inhibition rate is less than the critical inhibition rate, it isregarded as a negative sample.
When the sample inhibition rate is greater than or equal to the criticalinhibition rate, it is regarded as a positive sample.
3. This test method is not the only basis for diagnosis, it needs to bejudged in combination with clinical and other test methods.
Test Method Limitationsl
●This product is only used to detect the neutralizing antibodies of the newcoronavirus in human serum samples, and cannot be used to detect otherbody fluids.
●Due to the restriction of ELISA reaction principle, the negative test resultof this product cannot exclude the possibility of containing neutralizingantibody.
Explanation of Symbols |









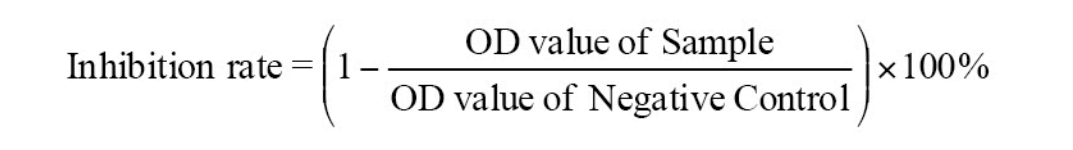 Critical inhibition rate =15%
Critical inhibition rate =15%








