[Reproduced] Dropping blood can detect the COVID-19! Zhong Nanshan's team successfully developed a 15-minute result test kit.
- Categories:Newsroom
- Author:
- Origin:
- Time of issue:2020-03-11
- Views:0
(Summary description)Nandu report :Recently, Zhong Nanshan's team successfully developed a technology which was tested by dripping, 15 minutes to issue results and IgM-IgG combined antibodies test kit for COVID-19. This kit can not only be used as a supplementary test for patients with negative viral nucleic acid tests to improve the clinical diagnosis rate ,but also as a means of rapid screening, indicating that a more convenient screening method will be come out soon !
[Reproduced] Dropping blood can detect the COVID-19! Zhong Nanshan's team successfully developed a 15-minute result test kit.
(Summary description)Nandu report :Recently, Zhong Nanshan's team successfully developed a technology which was tested by dripping, 15 minutes to issue results and IgM-IgG combined antibodies test kit for COVID-19. This kit can not only be used as a supplementary test for patients with negative viral nucleic acid tests to improve the clinical diagnosis rate ,but also as a means of rapid screening, indicating that a more convenient screening method will be come out soon !
- Categories:Newsroom
- Author:
- Origin:
- Time of issue:2020-03-11
- Views:0
Nandu report :Recently, Zhong Nanshan's team successfully developed a technology which was tested by dripping, 15 minutes to issue results and IgM-IgG combined antibodies test kit for COVID-19. This kit can not only be used as a supplementary test for patients with negative viral nucleic acid tests to improve the clinical diagnosis rate ,but also as a means of rapid screening, indicating that a more convenient screening method will be come out soon !
Zhong Nanshan's team published a research paper online in the Journal of Medical Virology on the 28th of this month. The reporter was informed that the paper described for the first time the development of SARS-CoV-2 IgM-IgG combined antibody test reagents and its application in the clinical diagnosis of COVID-19 infectious diseases. According to the team, the kit is convenient, fast, safe, and highly sensitive.
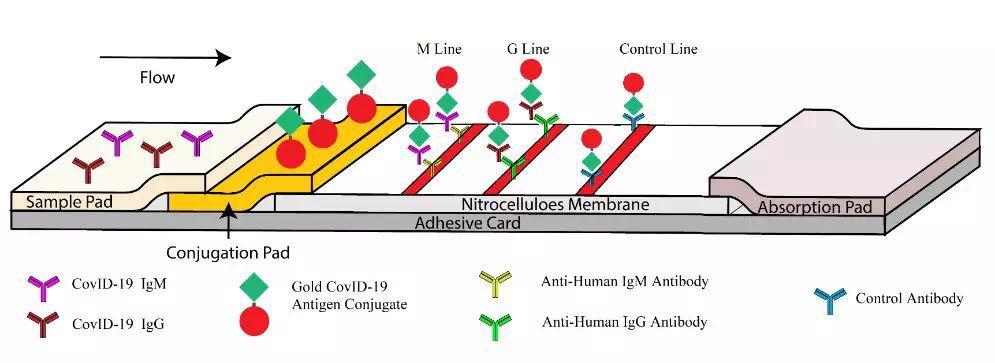
Schematic diagram of SARS-CoV-2 IgM-IgG combined antibody test reagent design
It is reported that the first unit of the thesis is the Guangzhou Respiratory Health Research Institute of the First Affiliated Hospital of Guangzhou Medical University, led by Zhong Nanshan, and it also unites Nanjing Second Hospital, Chongqing Public Health Medical Treatment Center, Huazhong University of Science and Technology Affiliated Union and Wuhan Red Cross Hui Hospital, the First Affiliated Hospital of Nanchang University, Wuhan First Hospital, Xi’an Jiaotong University First Affiliated Hospital, Guangdong Second People’s Hospital, Hunan Center for Disease Control and Prevention, and Jiangsu Meike Medical Technology Co., Ltd. formed a research team to jointly Complete the research. After hard work, the research team successfully developed a SARS-CoV-2 IgM-IgG combined antibody rapid detection reagent based on lateral immunochromatography technology.
The reagent has been tested on clinical specimens of COVID-19 patients in the above-mentioned well-known tertiary hospitals and provincial CDCs across the country, verifying the sensitivity and specificity of the reagent, as well as the value of clinical application. Compared with RT-PCR detection of viral nucleic acid that takes 3-4 hours to produce results, the detection reagent produces results in about 15 minutes, which greatly shortens the test time. After multi-center clinical specimen detection and evaluation, the sensitivity of the test reagent in clinical test is as high as 88.66%, and the test specificity is 90.63%; the sensitivity of IgM-IgG combined antibody detection is much higher than that of IgM or IgG single antibody test (sensitivity 94.83% vs 1.72% and 3.45%).
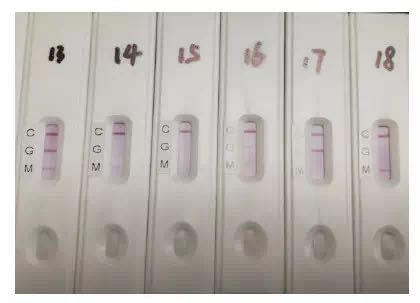
Schematic diagram of the results of SARS-CoV-2 IgM-IgG combined antibody test reagents in clinical specimens
According to the research team, the antibody test reagent uses fingertip blood and venous blood to compare the results, and found that the consistency of the two test is basically the same; it suggests that the SARS-CoV-2 IgM-IgG combined antibody rapid detection kit can be used as an immediate Test (POCT), you can take blood from your fingertips at the bedside. This will mean that there will be more convenient screening methods in the future. The research team said that they expect the research results and research products to play their due role in clinical diagnosis and primary screening, and they also look forward to taking advantage of screening during the period when large areas of personnel flow and gathering such as the resumption of work and school in the country.
Scan the QR code to read on your phone
- Email Us overseas@medomics-dx.com
- Call Us +86-025-58601060
- COVID-19 Solution
- Top
Contact Us
Phone: (+86) 025 - 58601060
E-mail: overseas@medomics-dx.com
Address:Building 01, Phase 6, No.71, Xinghui Road, Jiangbei New Area, Nanjing
- Email Us
- COVID-19 Solution
- Call Us (+86) 025 - 58601060
- top
Jiangsu Medomics Medical Technology Co,Ltd Powered By www.300.cn



![[Reproduced] Dropping blood can detect the COVID-19! Zhong Nanshan](/upload/s.png)






